COVID-19 testing may be administered through a local onsite program in your community or through your employer.
×

Our COVID-19 testing program supports the safe return of communities to work, school, public events, and other in-person interactions.
Below, you can learn more about the type of tests available and find additional information about collecting test samples and understanding your results.

Current tests can help identify whether you have an active COVID-19 infection, a previous infection, or presence of neutralizing antibodies from infection. Depending on the test, you may be asked to provide a nasal swab sample, a blood sample, or both.

Biodesix testing, in partnership with Bio-Rad Laboratories, GenScript Biotech, or other select vendors, is authorized for emergency use (EUA) by the Food and Drug Administration (FDA) to identify whether an individual has COVID-19 or antibodies indicating previous infection. These tests are only performed in qualified laboratories and have low rates of false positive and false negative results to ensure high quality and reproducibility.
COVID-19 testing may be administered through a local onsite program in your community or through your employer.
Typically, results are delivered securely through email or accessed through a mobile application on your phone.
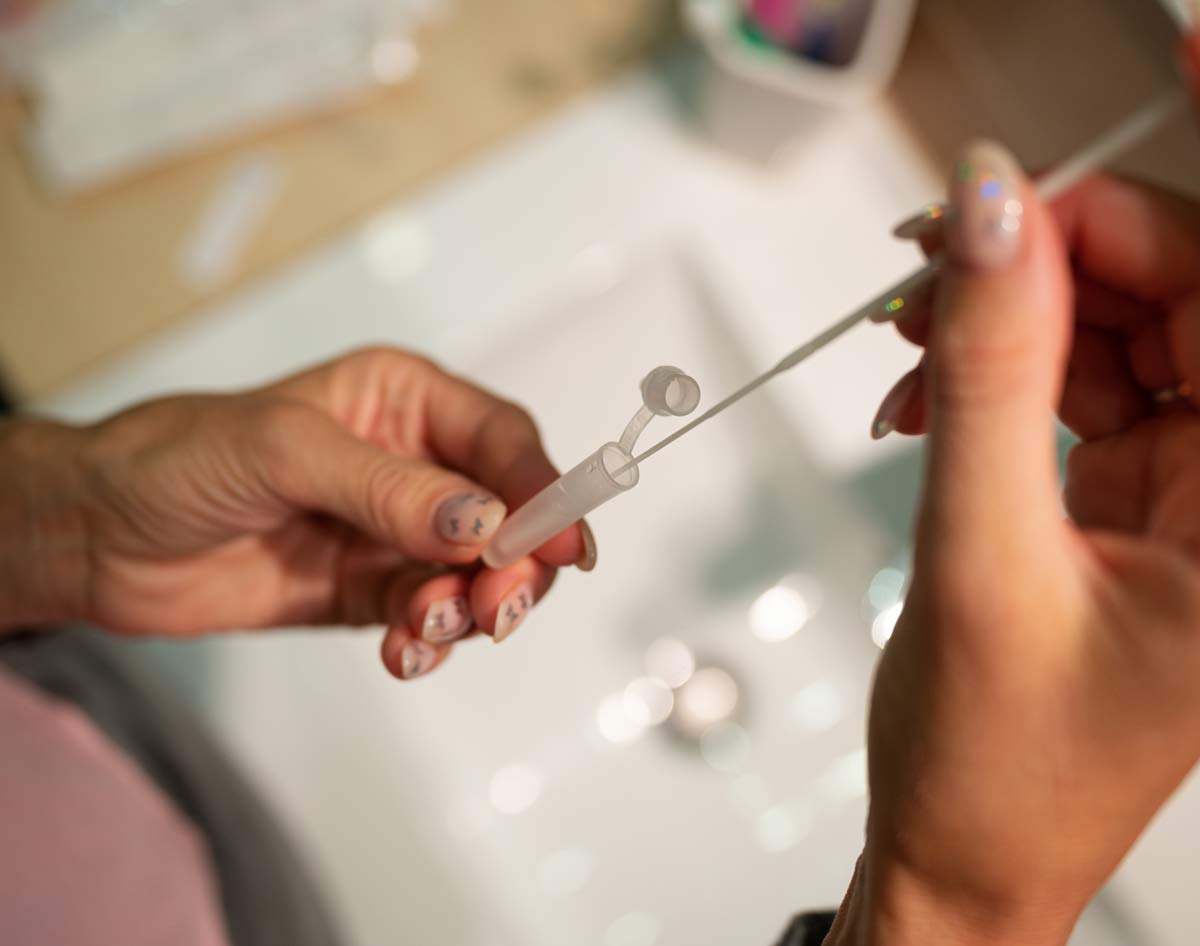
If your test requires you to provide a nasal swab sample, you will be tested to determine whether you may currently have COVID-19. These tests may be performed using PCR or rapid antigen technologies to find your result.
Tests may be administered through a local onsite program in your community or through your employer. Results will be made available to you as directed by the program. These results may be emailed to you or accessed through a mobile application on your phone.
Your test results will clearly state the specific test that was performed and the reported result, along with a brief explanation of what it means for you.
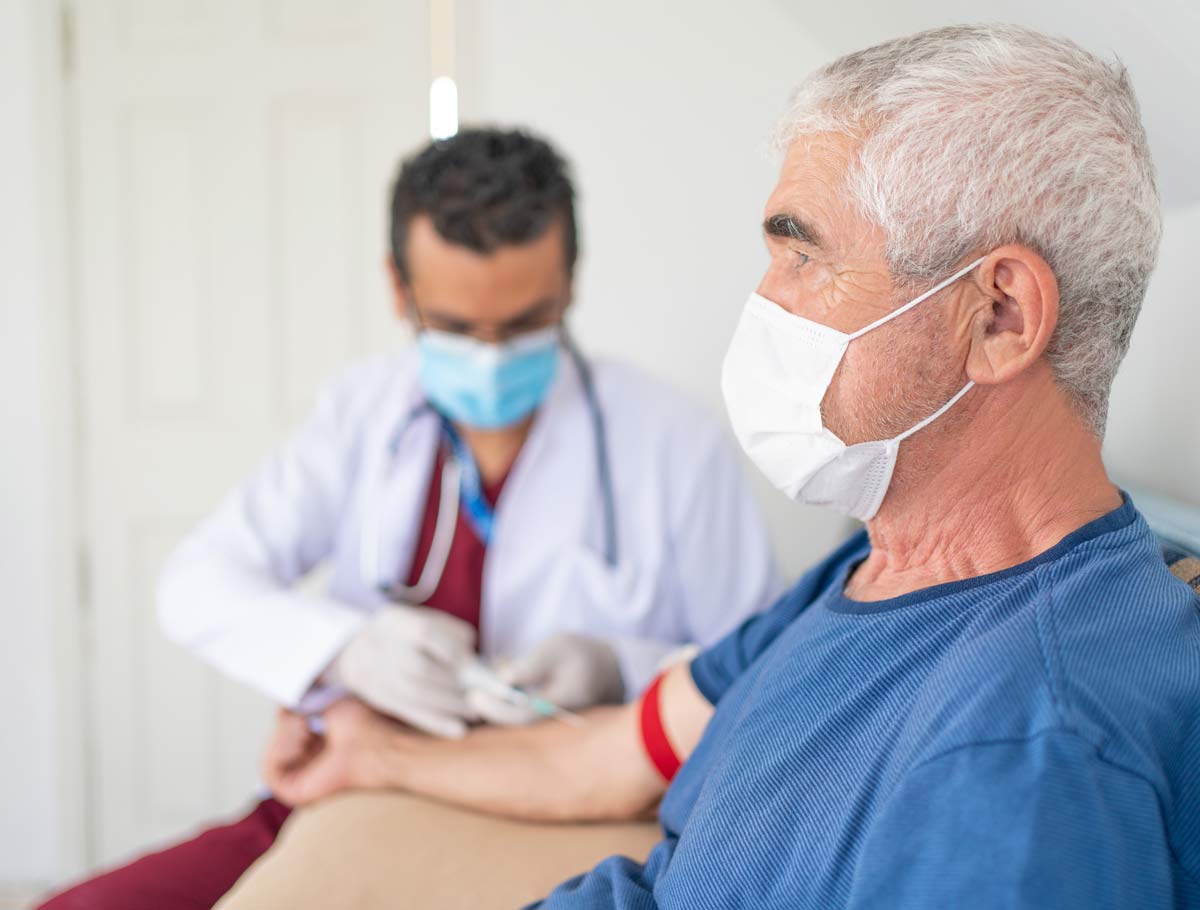
If you have been asked to submit a blood sample, you will be tested to find antibodies that indicate whether you may have a current or prior COVID-19 infection. Your test results will clearly state the specific test that was performed and the reported result, along with a brief explanation of what it means for you.
