
Accelerating Therapeutic Advancement Across Disease States
At Biodesix, we are well equipped to deliver precise molecular insights to drive the biopharmaceutical industry closer to therapeutic breakthrough. With a multi-omic approach backed by rigorous science, we can help answer the most complex questions throughout the clinical development process.
Biodesix understands the importance of robust quality and regulatory systems in commercializing a novel therapeutic. Through our own experience with five, on-market, clinical diagnostic tests and our two CAP-accredited, CLIA-certified, NYS CLEP-approved, and ISO 13485-certified U.S. based labs, Biodesix has the infrastructure and protocols to support sample logistics, clinical sample testing, regulatory documentation and submissions, and In Vitro Diagnostic (IVD) test development and approval (including CDx).
To bolster innovation, we provide comprehensive services powered by state-of-the-art genomic and proteomic technologies, applicable to multiple sample types. This includes, but is not limited to:
Biodesix has collaborated with 8 of the top 10 Pharmaceutical companies to turn questions into forward developmental progress1. Together, let’s continue to shape the future of precision medicine.
Connect with our team to further discuss your research needs.
Expose critical clinical development clues via longitudinal hot-spot monitoring of cell free DNA and RNA. Harness the power of LBx to determine clinical trial enrollment eligibility, optimize therapeutic dosages, measure efficacy endpoints (cfDNA/cfRNA clearance as an early indicator of PFS/OS), monitor the emergence of resistance mutations, and more through ddPCR™ or Next Generation Sequencing (NGS) genomic profiling.
Biodesix is deeply experienced in Droplet Digital™ PCR (ddPCR) testing through a standing relationship with Bio-Rad Laboratories. With an ultra-low limit of detection, low cost, and full quantification abilities, easily overcome noise and low sample volume challenges by utilizing ddPCR testing in your project. Many assays including KRAS, EGFRm, microsatellite instability (MSI), minimal residual disease (MRD), and more are pre-validated and readily available.
For target assays that are not off the shelf, Biodesix facilitates custom development and validation. Dive deeper into our assay offerings here.
ULTRA LOW LIMIT OF DETECTION
LOW COST
FULL QUANTIFICATION ABILITIES
RAPID TURN AROUND TIME
Next generation sequencing at Biodesix is powered by a strong partnership with Thermo Fisher Scientific to deliver the latest science. Utilizing a fully integrated workflow, we are able to provide industry leading turn-around times.
Again, for target NGS assays that are not off the shelf, Biodesix facilitates custom development and validation.
Contact our team for full details on the laboratory technologies and how they can power your project.
Read more on Biodesix’s ctDNA work demonstrating early clearance of EGFRm ctDNA as a predictor of outcome.
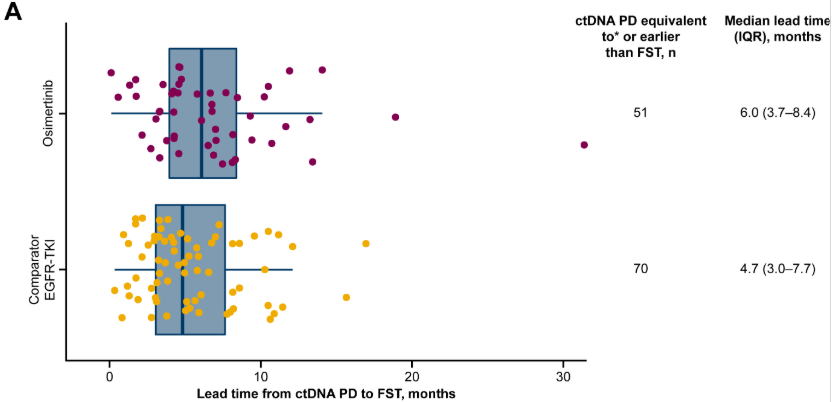

This image is used under Open Access. Credit: Gray, Jhanelle E. et al. Longitudinal Analyses of Circulating Tumor DNA for the Detection of EGFR Mutation-Positive Advanced NSCLC Progression During Treatment: Data From FLAURA and AURA3. Journal of Thoracic Oncology, Volume 19, Issue 11, 1525 – 1538
Gather comprehensive proteomic insights from biological samples through discovery proteomics. Characterize protein-protein interactions, post-translational modifications and differential protein expression. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) provides a window into the plasma proteome for biomarker discovery, facilitates the development of protein-based classifiers and enables next-generation spatial omics.
Looking to go deeper? Untargeted liquid chromatography tandem MS (LC-MS/MS) allows for the unbiased identification and quantification of thousands of protein targets in a single liquid biopsy, tissue or cell culture sample.
Contact our team for full details on the laboratory technologies and how they can power your project.
See more on how Biodesix uses targeted and untargeted proteomics.
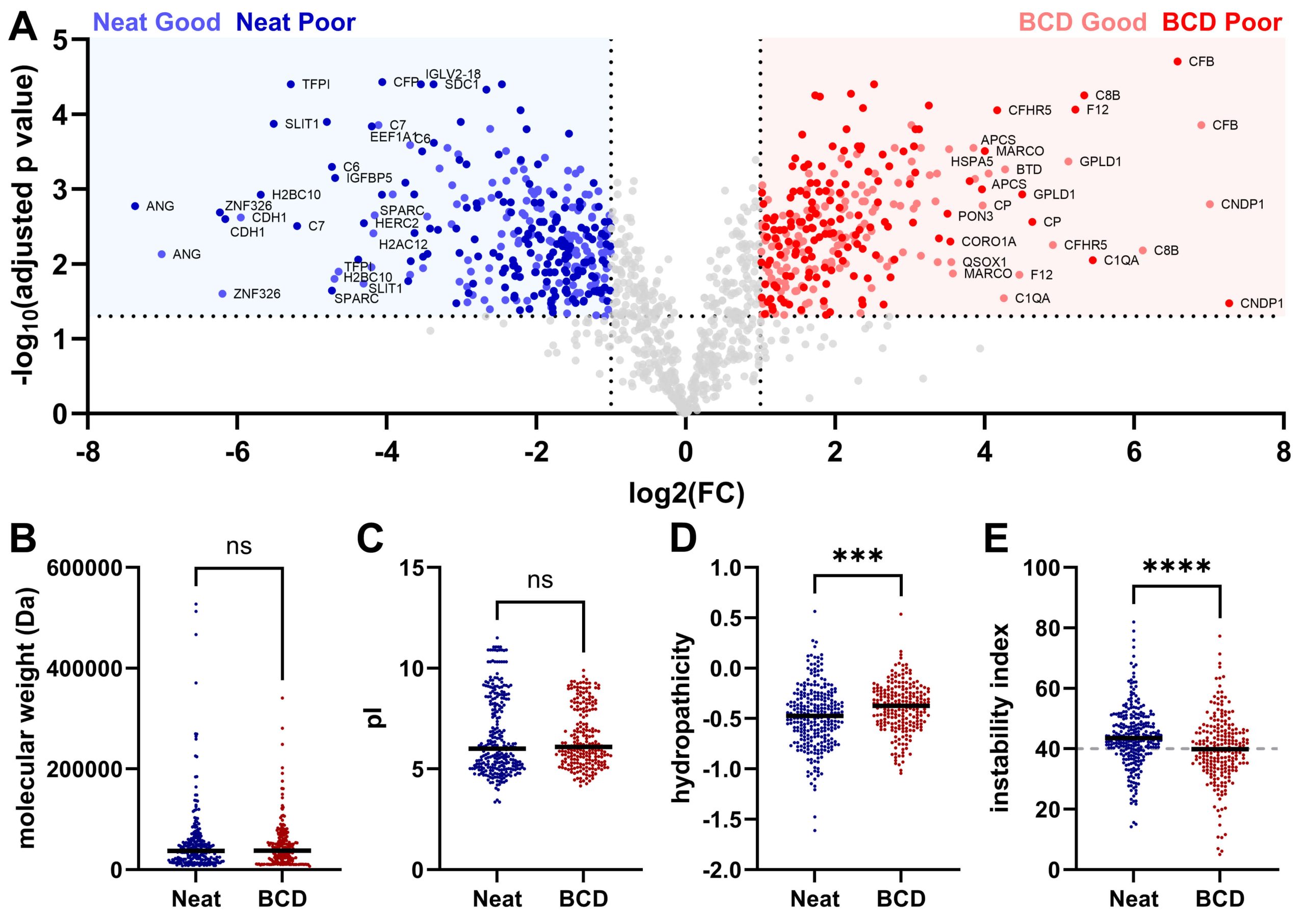

This image is used under Open Access. Credit: McDowell CT, Weaver AL, Vargas-Cruz N, Kaiser NK, Nichols CM, Pestano GA. Use of a Novel Whole Blood Separation and Transport Device for Targeted and Untargeted Proteomics.Biomedicines. 2024 Oct 11;12(10):2318. doi: 10.3390/biomedicines12102318. PMID: 39457630; PMCID: PMC11504527.
Evaluate the abundance of proteins associated with your drug’s mechanism of action or the patient’s therapeutic response over time. Biodesix can provide absolute quantification of proteins of interest by targeted LC-MS/MS for biomarker validation, minimal residual disease (MRD), monitoring drug response, targeted protein degradation (TPD), disease mechanism, pharmacokinetic/pharmacodynamic (PKPD) and cell signaling studies. Targeted LC-MS/MS assays are easily transferrable to an enzyme-linked immunosorbent assay (ELISA) format for scalability and cost-effectiveness.
Contact our team for full details on the laboratory technologies and how they can power your project.
Biodesix’s infrastructure stemming from success in clinical diagnostics bolsters the ability to support your project end to end. With an FDA registered manufacturing facility in house, Biodesix can customize prospective collection kits to your needs that meet all regulatory requirements. We also routinely partner with central labs and clinical trial sites to facilitate the management and procurement of samples.
Once samples arrive on site, Biodesix is well equipped to properly handle them. We have a robust quality management system to properly procure and manage samples, as well as maintain an appropriate chain of custody for all samples and associated data.


References:
- “The top 20 pharma companies by 2023 revenue;” Fierce Pharma; April 2024. https://www.fiercepharma.com/pharma/top-20-pharma-companies-2023-revenue
- Droplet Digital and ddPCR are trademarks of Bio-Rad Laboratories, Inc.