×
Biodesix has the expertise, experience, and evidence to be your trusted biomarker discovery and development partner. We go beyond contract research and partner with you to discover biomarkers using a multi-omic approach along with our proprietary machine learning platform.
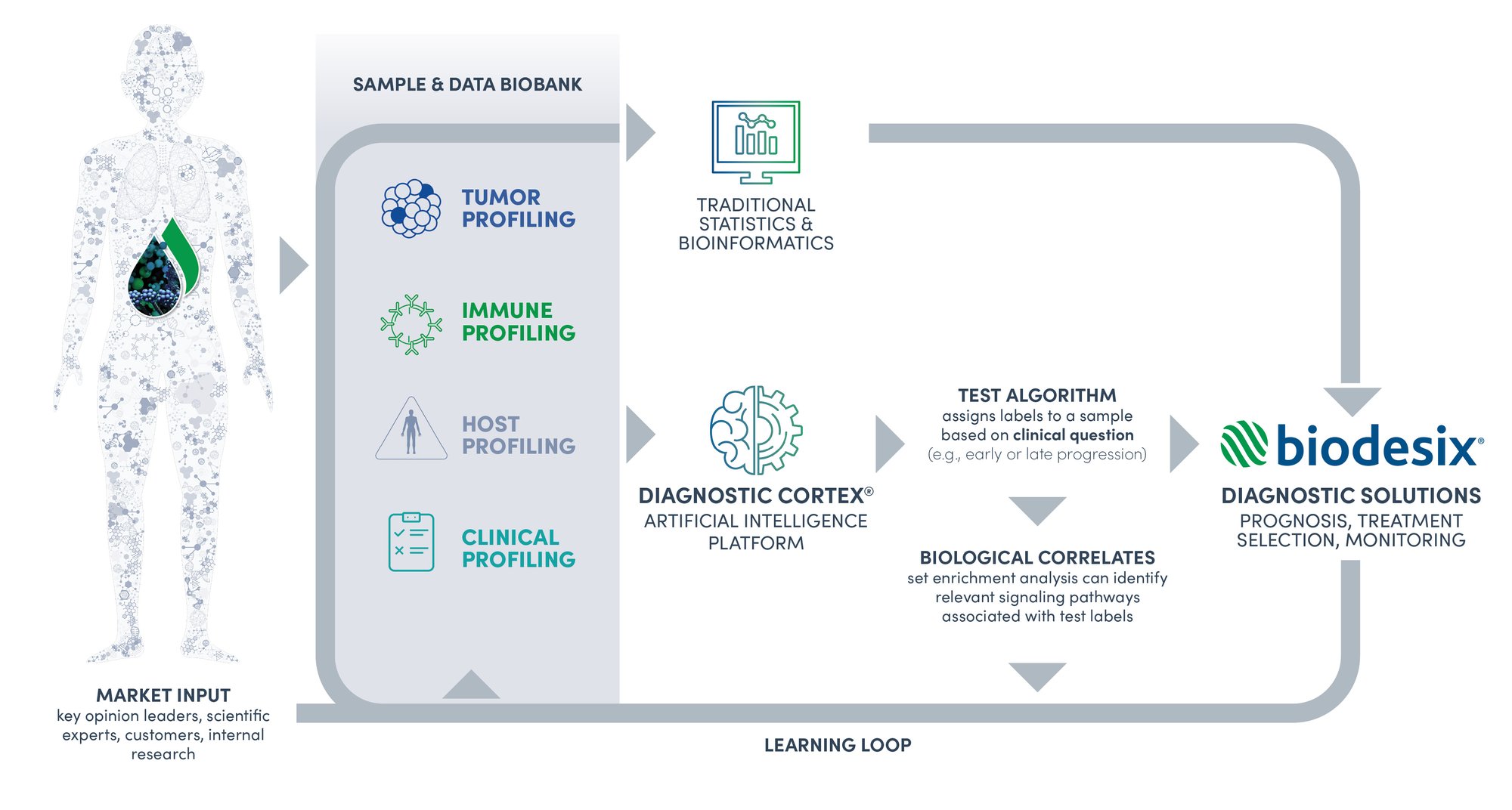
Our core belief is that no single technology will answer all diagnostic research questions that we encounter. We utilize multiple technology platforms combined with our transparent AI for diagnostic research to uncover novel insights into the disease biology of biopharmaceutical therapeutics in clinical development. Our services support known target feasibility studies as well as unbiased diagnostic research to discover novel biomarkers of interest.
Our strong expertise in genomic and proteomic assay development, powered by our proprietary AI platform, enable our BioPharma partners to accelerate development and commercialization timelines.
Development options range from: research assay development through to design controlled product development process. Our established product development process is designed to be compliant with Quality and Regulatory standards while meeting specific project requirements.
We enable success for partners through our broad capabilities and areas of expertise, which include:

In addition to traditional statistical methods used for known biomarker discovery, we employ a unique approach to discover innovative diagnostic tests. Our proprietary Diagnostic Cortex® AI platform generates insights using biomarker data related to the tumor, immune response, and host-status along with clinical factors. This enables us to interpret the holistic disease state of each patient or clinical dataset we encounter.