REAL WORLD DATA: Performance of Blood-Based Proteomic Profiling in Immunotherapy-Treated Advanced Stage NSCLC Patients
Rich (Thompson), P et al. 2019 (Multidisciplinary Thoracic Cancers Symposium) & Chuang, Y et al. 2019 (RAS Targeted Drug Discovery Symposium)
Introduction: The VeriStrat® proteomic test was designed using mass spectrometry combined with our proprietary artificial intelligence platform. The test provides a personalized view of each patient's disease state by measuring acute phase proteins and the acute phase response which indicates chronic inflammation and a more aggressive cancer.
Methods: The INSIGHT study includes 33 US sites having enrolled 2700 patients to date in a registry-style design allowing NSCLC patients with any stages of disease or lines of therapy to enroll. Patient characteristics, therapeutic decisions, staging, disease monitoring metrics, and available biomarker data are being collected and patients are being followed for 18 months. Patients are receiving blood-based VeriStrat® proteomic testing prior to therapy initiation. Testing was performed in the Biodesix® CAP/CLIA/NYS CLEP/ISO13485-approved lab in Boulder, CO. An interim analysis of the INSIGHT patients with at least one year of follow up was performed. A total of 980 patients were included in the INSIGHT interim analysis.
Results: Based on the current interim analysis results, the VeriStrat test was predictive of overall survival in immunotherapy treated patients at all lines of therapy in advanced NSCLC, even when adjusted for PD-L1 status in a multi-variate analysis (p<0.0001)1,2.
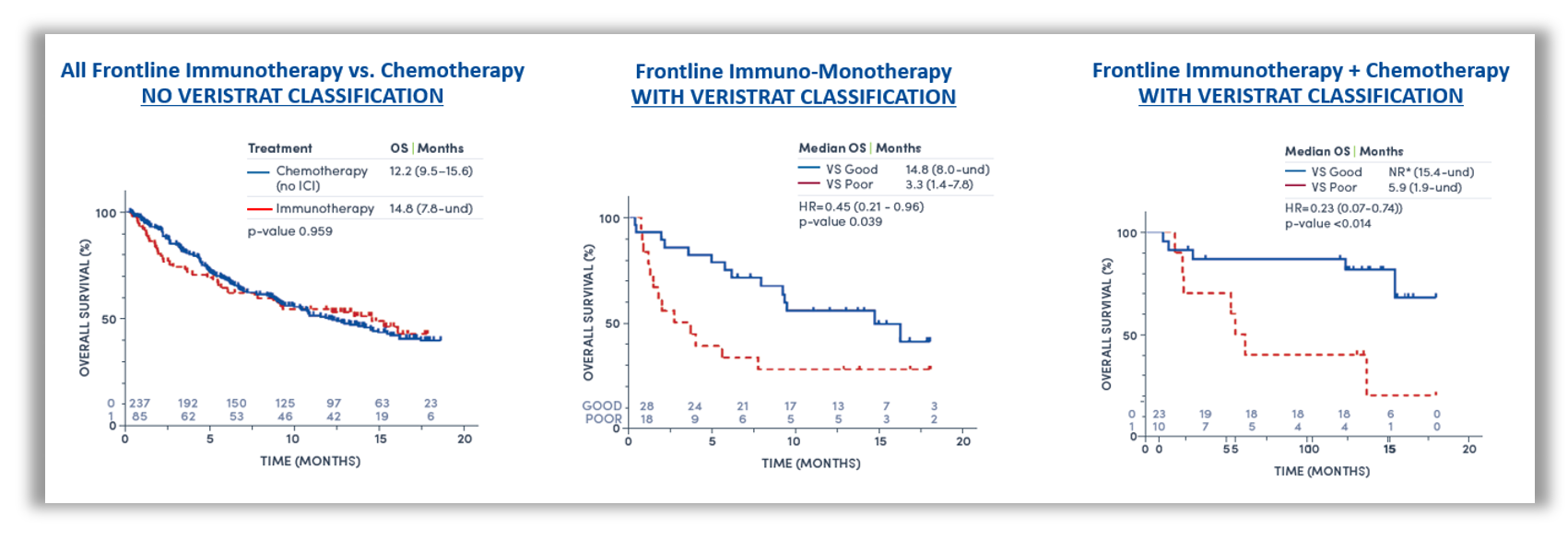
Figure 1: Kaplan-Meier plot of OS by treatment regimen. All frontline immunotherapy regimens versus chemotherapy without VeriStrat classification (left), frontline immuno-monotherapy with VeriStrat classification (middle), frontline immunotherapy + chemotherapy with VeriStrat classification (right)1.
KRAS mutations are known to be associated with a poorer prognosis for patients with NSCLC and until recently, there was no treatment targeted at KRAS mutations. Combining blood-based proteomic and genomic testing provides more complete information on a patient’s prognosis and can help guide immunotherapy treatment decisions.
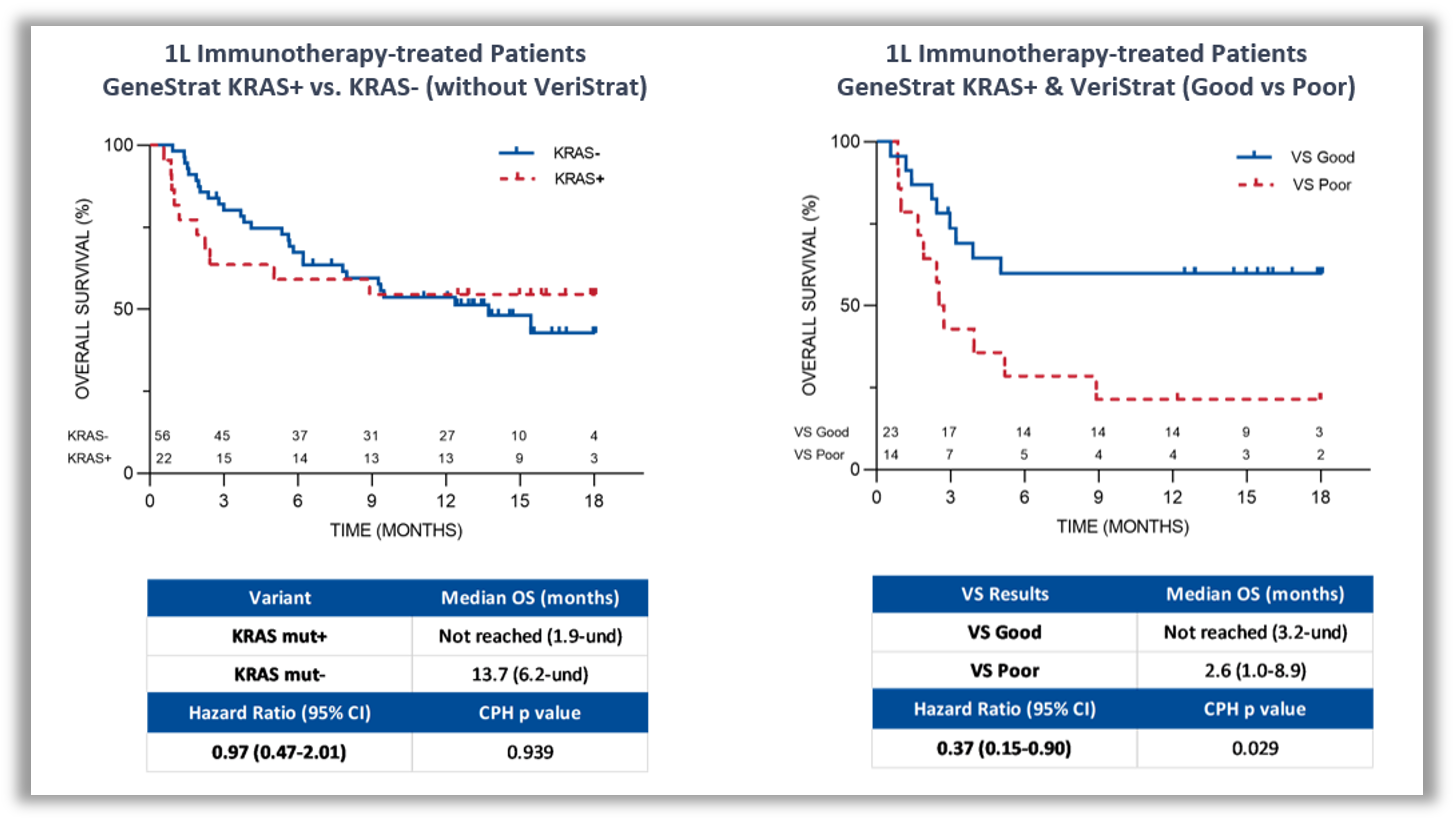
Figure 2: Kaplan-Meier plot of OS in frontline immunotherapy-treated patients. Frontline immunotherapy-treated patients classified by ctDNA KRAS mutation status (left). Frontline immunotherapy-treated patients with ctDNA KRAS mutation positive status classified by the VeriStrat test (right)2.
If you are interested in learning more about the VeriStrat proteomic test, please contact us.
References:

